- FORUM
- PROJECTS
- ABOUT US
- RESOURCES
- CONTACT US
- FORUM
- PROJECTS
- ABOUT US
- RESOURCES
- CONTACT US
Ferrous Metal and Alloy
Classification of Metal and Alloys Ferrous – Iron as base metal Nonferrous – No iron in composition Alloy definitions –
- Combination of two or more elements.
- Combination of two or more metallic elements.
Steel : Historic Overview
- round 800 B.C. -: the general use of iron
- 16th-19th century -: wrought iron / cast iron
- round 1870 A.D. -: the first production of modern steel
- late 19th century -: multi-storey iron structure buildings
- early 20th century -: the use of tower and mobile cranes in construction
Metals
Ferrous Metals -: Cast irons, Steels.
Super alloys -: Iron-based, Nickel-based, Cobalt-based.
Non-ferrous metals -: Aluminum and its alloys Copper and its alloys, Magnesium and its alloys, Nickel and its alloys, Titanium and its alloys, Zinc and its alloys, Lead & Tin, Refractory metals, Precious metals.
Alloys In Metals
Compounds
- Chemically bonded
- Distinct from its constituents
Mixtures
- Not chemically bonded
- Retains individual identity of ingredients
Solutions
- Liquid
- Solid
Properties of metal & alloy
Mechanical properties
Strength, Toughness, Hardness, Ductility, Elasticity, Fatigue and Creep
Physical properties
Density, Specific heat, Melting and boiling point, Thermal expansion and conductivity, Electrical and magnetic properties
Chemical properties
Oxidation, Corrosion, Flammability, Toxicity, …
Mechanical Properties Of Metals
Strength
The ability of a material to stand up to forces being applied without it bending, breaking, shattering or deforming in any way..
ElasticityThe ability of a material to absorb force and flex in different directions, returning to its original position.

PlasticityThe ability of a material to be change in shape permanently.
DuctilityThe ability of a material to change shape (deform) usually by stretching along its length..
Tensile StrengthThe ability of a material to stretch without breaking or snapping.
Malleability The ability of a material to be reshaped in all directions without cracking.
ToughnessA characteristic of a material that does not break or shatter when receiving a blow or under a sudden shock.
Conductivity The ability of a material to conduct electricity..
HardnessThe ability of a material to resist scratching, wear and tear & indentation.

FatigueFatigue failures occur when metal is subjected to a repetitive or fluctuating stress and will fail at a stress much lower than its tensile strength.
- Fatigue failures occur without any plastic deformation (no warning).
- Fatigue surface appears as a smooth region, showing beach mark or origin of fatigue crack.
FusibilityFusibility is defined as the ability of a metal to become liquid by the application of heat. Metals are fused in welding. Steels fuse at approximately 2,500°F, and aluminum alloys at approximately 1,110°F.
CreepThe mechanical strength of metals decreases with increasing temperature and the properties become much more time dependent. Metals subjected to a constant load at elevated temperatures will undergo ‘creep’, a time dependent increase in length.
Creep in metals is defined as time dependent plastic deformation at constant stress (or load) and temperature.
Strength
- Strength is the property that enables a metal to to resist deformation under load The ultimate strength is the maximum strain a material can withstand.
- Tensile strength is a measurement of the resistance to being pulled apart when placed in a tension load.
- Fatigue strength is the ability of material to resist various kinds of rapidly changing stresseS and is expressed by the magnitude of alternating stress for a specified number of cycles.
- Impact strength is the ability of a metal to resist suddenly applied loads and is measured in foot-pounds of force.
Hardness

Hardness is the property of a material to resist permanent indenation. Because there are several methods of measuring hardness, the hardness of a material is always specified in terms of the particular test that was used to measure this property. Rockwell, Yickers, or Brinell are some of the methods of testing. Of these tests, Rockwell is the one most frequently used.
Toughness
- Toughness is the property that enables a material to withstand shock and to be deformed without rupturing .
- Toughness may be considered as a combination of. strength and plasticity.
- It is metal ability to absorb energy before fracture. Recall that ductility is a measure of how much something deforms plastically before fracture, but just because a material is ductile does not make it tough.
- The ability of a metal to deform plastically and to absorb energy in the process before fracture is termed toughness.
- The key to toughness is a good combination of strength and ductility. A material with high strength and high ductility will have more toughness than a material with low strength and high ductility. Therefore, one way to measure toughness is by calculating the area under the stress strain curve from a tensile test. This value is simply called “material toughness” and it has units of energy per volume. Material toughness equates to a slow absorption of energy by the material.
Elasticity

When a material has a load applied to it, the load causes the material to deform. Elasticity is the ability of a material to return to its original shape after the load is removed. Theoretically, the elastic limit of a material is the limit to which a material can be loaded and still recover its original shape after the load is removed.
Plasticity
- Plasticity is the ability of a material to deform permanently without breaking or rupturing.
- This property is the opposite of strength. By careful alloying of metals, the combination of plasticity and strength is used to manufacture large structural members. For example, should a member of a bridge structure become overloaded, plasticity allows the overloaded member to flow allowing the distribution of the load to other parts of the bridge structure.
Brittleness
- Brittleness is the opposite of the property of plasticity.
- A brittle metal is one that breaks or shatters before it deforms. White cast iron and glass are good examples of brittle material.
- Generally, brittle metals are high in compressive strength but low in tensile strength. As an example, you would not choose cast iron for fabricating support beams in a bridge.
Ductility and Malleability
Ductility is the property that enables a material to stretch, bend or twist without cracking or breaking. This property makes it possible for a material to be drawn out into a thin wire. In comparison, malleability is the property that enables a material to deform by compressive forces without developing defects. A malleable material is one that can be stamped, hammered, forged, pressed, or rolled into thin sheets.
Corrosion Resistance
- Corrosion resistance, although not a mechanical property, is important in the discussion of metals.
- Corrosion resistance is the property of a metal that gives it the ability to withstand attacks from atmospheric, chemical, or electrochemical conditions. Corrosion, sometimes called oxidation, is illustrated by the rusting of iron.
- Table 1-2 lists four mechanical properties and the corrosion resistance of various metals or alloys. The first metal or alloy in each column exhibits the best characteristics of that property. The last metal or alloy in each column exhibits the least. In the column labelled “Toughness,” note that iron is not as tough as copper or nickel; however, it is tougher than magnesium, zinc, and aluminium. In the column labelled “Ductility,” iron exhibits a reasonable amount of ductility; however, in the columns labelled “Malleability” and “Brittleness,” it is last.
Metal Types
- The metals that Builders work with are divided into two general classifications: Ferrous and nonferrous.
- Ferrous metals are those composed primarily of iron and iron alloys.
- Nonferrous metals are those composed primarily of some element or elements other than iron.
- Nonferrous metals or alloys sometimes contain a small amount of iron as an alloying element or as an impurity.
Ferrous Metals
- The word is derived from the Latin word ferrum “iron”).
- Ferrous metals include all forms of iron and steel alloys. A few examples include wrought iron, cast iron, carbon steels, alloy steels, and tool steels. Ferrous metals are iron-base alloys with small percentages of carbon and other elements added to achieve desirable properties. Normally, ferrous metals are magnetic and nonferrous metals are nonmagnetic.
Iron
Pure iron rarely exists outside of the laboratory. Iron is produced by reducing iron ore to pig iron through the use of a blast furnace. From pig iron many other types of iron and steel are produced by the addition or deletion of carbon and alloys.

Iron Ores
- Iron ores are rocks and minerals from which metallic iron can be economically extracted.
- The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, deep purple, to rusty red.
- The iron itself is usually found in the form of magnetite (Fe3O4), hematite (Fe2O3), goethite (FeO(OH)), limonite (FeO(OH).n(H2O)) or siderite (FeCO3).
- Hematite is also known as “natural ore”, a name which refers to the early years of mining, when certain hematite ores containing up to 66% iron could be fed directly into iron-making blast furnaces.
- Iron ore is the raw material used to make pig iron, which is one of the main raw materials to make steel.
- 98% of the mined iron ore is used to make steel. Indeed, it has been argued that iron ore is “more integral to the global economy than any other commodity, except perhaps oil.


Pig Iron
Pig iron is composed of about 93% iron, from 3% to 5% carbon, and various amounts of other elements. Pig iron is comparatively weak and brittle
Wrought Iron; therefore, it has a limited use and approximately 90% produced is refined to produce steel. Cast-iron pipe and some fittings and valves are manufactured from pig iron. Contains 0.15% carbon
- wrought iron to resist corrosion and oxidation.
- The chemical analyses of wrought iron and mild steel are just about the same. The difference comes from the properties controlled during the manufacturing process.
- Wrought iron can be gas and arc welded, machined, plated, and easily formed; however, it has a low hardness and a low-fatigue strength.
- It can be molded easily and has good resistance to corrosion.
- Wrought iron is used extensively where corrosion resistance is needed.
- It’s ductility is lower than steel.
- It’s tensile strength is lower.

Cast Iron
- Cast iron is any iron containing greater than 2% carbon alloy.
- Manufactured by reheating pig iron (in a cupola) and blending it with other material of known composition.
- Alternate layers of pig iron (with or without scrap steel) and coke are charged into furnace.
- Limestone is added to flux the ash from the coke.
- Heat necessary for the smelting is supplied by the . combustion of coke and air supplied by the blast.
- Cupola function to purify iron and produce a more uniform product.
- When sufficient metal is accumulated at the bottom of the furnace, it is tapped.
- Cast iron has a high-compressive strength and good wear resistance; however, it lacks ductility, malleability, and impact strength. Alloying it with nickel, chromium, molybdenum, silicon, or vanadium improves toughness, tensile strength, and hardness. A malleable cast iron is produced through a prolonged annealing process.


Ingot Iron
Ingot iron is a commercially pure iron (99.85% iron) that is easily formed and possesses good ductility and corrosion resistance. The chemical analysis ‘and properties of this iron and the lowest carbon steel are practically the same. The lowest carbon steel, known as dead-soft, has about 0.06% more carbon than ingot iron. In iron the carbon content is considered an impurity and in steel it is considered an alloying element. The primary use for ingot iron is for galvanized and enameled sheet.
Steel
- Contains up to 1.5% of Carbon (Specific Gravity 7.7)
- Of all the different metals and materials that we use in our trade, steel is by far the most important. When steel was developed, it revolutionized the American iron industry. With it came skyscrapers, stronger and longer bridges, and railroad tracks that did not collapse. Steel is manufactured from pig iron by decreasing the amount of carbon and other impurities and adding specific amounts of alloying elements.
- Do not confuse steel with the two general classes of iron: cast iron (greater than 2% carbon) and pure iron (less than 0.15% carbon). In steel manufacturing, controlled amounts of alloying elements are added during the molten stage to produce the desired composition.
- Carbon in excess of 1.5% does not combine with iron , but will be present as free graphite , Thus the dividing line of cast iron and steel is presence of free graphite . If there is free graphite then it is cast iron, otherwise it is steel.
- Steel and wrought iron can be distinguished by putting a drop of nitric acid on metal , steel will produce a gray stain due to higher carbon content.
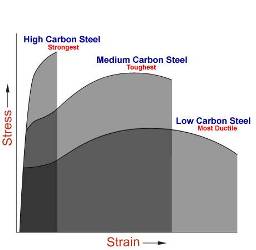
Carbon Steel
- Carbon steel is a term applied to a broad range of steel that falls between the commercially pure ingot iron and the cast irons. This range of carbon steel may be classified into four groups:
- Low-Carbon Steel 0.05% to 0.30% carbon
- Medium-Carbon Steel 0.30% to 0.45% carbon
- High-Carbon Steel 0.45% to 0.75% carbon
- Very High-Carbon Steel 0.75% to 1.70% carbon
Low-carbon Steel

Steel in this classification is tough and ductile, easily machined, formed, and welded. It does not respond to any form of heat treating, except case hardening.
Medium-carbon Steel

These steels are strong and hard but cannot be welded or worked as easily as the low-carbon steels. They are used for crane hooks, axles, shafts, setscrews, and so on.
High-carbon Steel
Steel in these classes respond well to heat treatment and can be welded. When welding, special electrodes must be used along with preheating and stress-relieving procedures to prevent cracks in the weld areas. These steels are used for dies, cutting tools, mill tools, railroad car wheels, chisels, knives, and so on.

Stainless Steel
- This type of steel is classified by the American Iron and Steel Institute (AISI) into two general series named the 200-300 series and 400 series. Each series includes several types of steel with different characteristics.
- The 200-300 series of stainless steel is known as AUSTENITIC. This type of steel is very tough and ductile in the welded condition; therefore, it is ideal for welding and requires no annealing under normal atmospheric conditions. The most well-known types of steel in this series are the 302 and 304. They are commonly called 18-8 because they are composed of 18% chromium and 8% nickel. The chromium nickel steels are the most widely used and are normally nonmagnetic.
- Characterized by their corrosion resistance, high strength and ductility, and high chromium content.
- Stainless as a film of chromium oxide protects the metal from corrosion.
- Five types of stainless steels:
- Austenitic steels
- Ferritic steels
- Martensitic steels
- Precipitation-hardening (PH) steels
- Duplex-structure steels
Alloy Steels
Steels that derive their properties primarily from the presence of some alloying element other than carbon are called ALLOY STEELS.
One or more of these elements may be added to the steel during the manufacturing process to produce the desired characteristics. Alloy steels may be produced in structural sections, sheets, plates, and bars for use in the “as-rolled” condition.
- Nickel Steel
- Chromium Steel
- Chrome Vanadium Steel
- Tungsten Steel
- Manganese Steel

Nickel Steels
These steels contain from 3.5% nickel to 5% nickel. The nickel increases the strength and toughness of these steels. Nickel steel’ containing more than 5% nickel has an increased resistance to corrosion and scale. Nickel steel is used in the manufacture of aircraft parts, such as propellers and airframe support members.
Chromium Steels

These steels have chromium added to improve hardening ability, wear resistance, and strength. These steels contain between 0.20 % to 0.75% chromium and 0.45% carbon or more. Some of these steels are so highly resistant to wear that they are used for the races and balls in antifriction bearings. Chromium steels are highly resistant to corrosion and to scale.
Chrome Vanadium Steel
This steel has the maximum amount of strength with the least amount of weight. Steels of this type contain from 0.15% to 0.25% . vanadium, 0.6% to 1.5% chromium, and 0.1 % to 0.6% carbon. Common uses are for crankshafts, gears, axles, and other items that require high strength. This steel is also

used in the manufacture of high-quality hand tools, such as wrenches and sockets.
Tungsten Steel

This is a special alloy that has the property of red hardness. This is the ability to continue to cut after it becomes red-hot. Because this alloy is expensive to produce, its use is largely restricted
Manganese Steels to the manufacture of drills, lathe tools, milling cutters, and similar cutting tools.
The amount of manganese used depends upon the properties desired in the finished product. Small amounts of manganese produce strong, free-machining steels. Larger amounts (between 2% and 10%) produce somewhat brittle steel, while still larger amounts (11% to 14%) produce a steel that is tough and very resistant to wear after proper heat treatment. Railroad tracks, for example, are made with steel that contains manganese
Iron And Steel Manufacturing Process
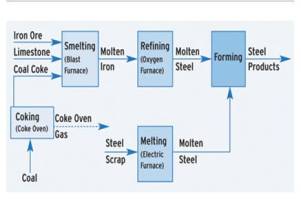
Manufacture Of Steel : Bessemer Process
The Bessemer process was the first inexpensive industrial process for the mass- production of steel from molten pig iron. The process is named after its inventor, Henry Bessemer, who took out a patent on the process in 1855. The key principle is removal of impurities from the iron by oxidation with air being blown through the molten iron. The oxidation also raises the temperature of the iron mass and keeps it molten.
Manufacture Of Steel Open Hearth Process
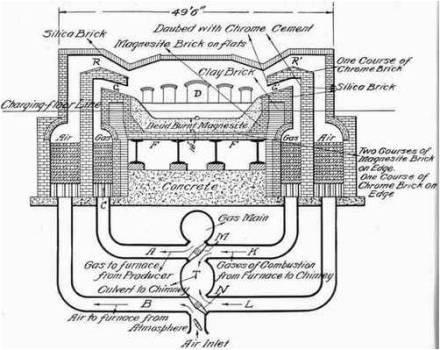
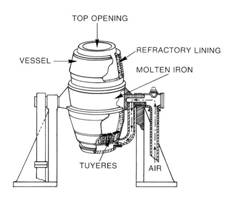
Manufacture Of Steel L D Process (Basic Oxygen Process)
In the basic oxygen process, steel is also refined in a pear-shaped furnace that tilts sideways for charging and pouring. Air, however, has been replaced by a high-pressure stream of nearly pure oxygen. After the furnace has been charged and turned upright, an oxygen lance is lowered into it. The water-cooled tip of the lance is usually about 2 m (about 6 ft) above the charge although this distance can be varied according to requirements. Thousands of cubic meters of oxygen are blown into the furnace at supersonic speed. The oxygen combines with carbon and other unwanted elements and starts a high-temperature churning reaction that rapidly burns out impurities from the pig iron and converts it into steel.
Production Of Steel
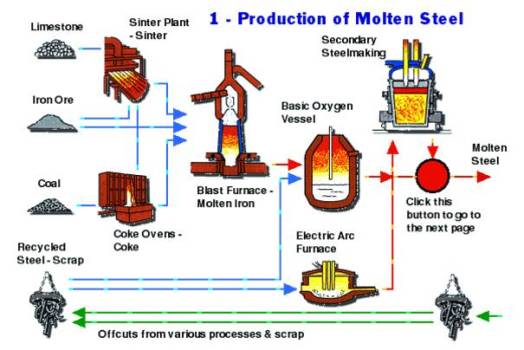
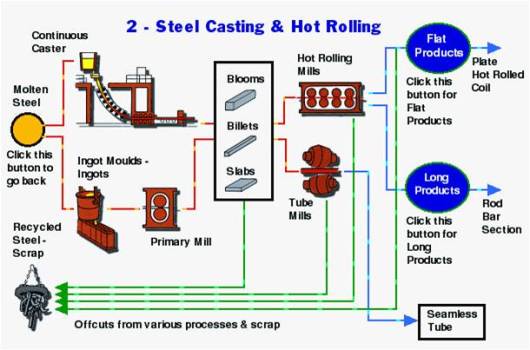
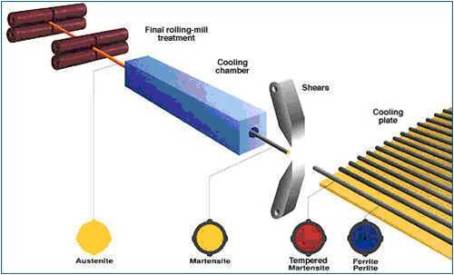
Production Of TMT Bars
Tempcore: TMT BARS are removed from cooling zone. A temprature gradient is established in the cross section. It causes heat to flow from centre to surface. The martensite left at centre is tempered by heat flow. So it is known as tempcore. After the intensive cooling, the tmt bar is exposed to air and the core reheats the quenched surface layer by conduction, therefore tempering the external martensite. helps them attain a higher yield strength.
Basic Types of Tool and Die Steels
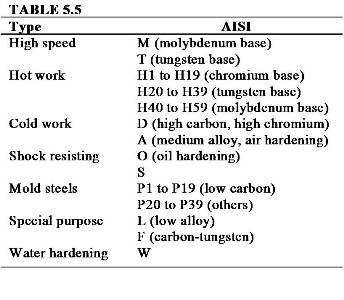
Metal Identification
Steel Numbering Systems
- SAE – society of automotive engineers
- AISI – American iron and steel institute
- C = carbon
- Cr = chromium
- Mn = Manganese
- Mo = Molybdenum
- Ni = Nickel
- Si = Silicon
- V = Vanadium
- First number indicates type of steel
- Second number approx. % of dominate element
- Third and fourth digit denotes % of carbon in hundredths
- 1018 = Carbon steel with .18% of carbon content
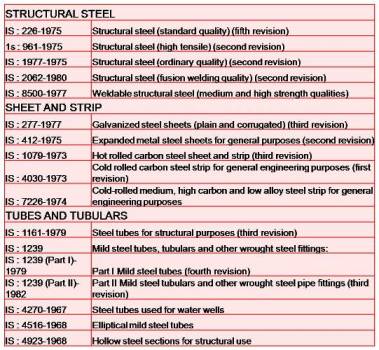
IS CODE :
Forum Threads
Study Notes PDF
study notes of FERROUS METAL & ALLOY Construction Material
construction material- Ferrous metal.pdf
Register in Front Desk Architects & Planners Forum to download above PDF
Disclaimer
Information on this site is purely for education purpose. The materials used and displayed on the Sites, including text, photographs, graphics, illustrations and artwork, video, music and sound, and names, logos, IS Codes, are copyrighted items of respective owners. Front Desk is not responsible and liable for information shared above.
References
1) Metals Processing Advisor, “Iron and Steel Overview: An in depth look at the Iron and Steel Industries and associated process equipment.”
2) World-Bank. “Industrial Pollution Prevention and Abatement: Coke Manufacturing.” Washington, D.C. July 1998.
External Links
chestofbooks.com/crafts/mechanics/Mechanical-Processes/120-The-Open-Hearth-Furnace.html
For your views and discussions Register in FDAchitects Forum …
1 Comment
You had done an awesome research of ferrous metal