- FORUM
- PROJECTS
- ABOUT US
- RESOURCES
- CONTACT US
- FORUM
- PROJECTS
- ABOUT US
- RESOURCES
- CONTACT US
Non Ferrous Metal and Alloy
Nonferrous metals contain either no iron or only insignificant amounts used as an alloy. Some of the more common nonferrous metals Steelworkers work with are as follows: copper, brass; bronze, copper-nickel alloys, lead, zinc, tin, aluminium, and Duralumin. These metals are nonmagnetic.
Non-Ferrous Metals: Alloys
Brass (Copper+Zinc)
- Yellow colour
- Very ductile and malleable
- Good tensile endurance


Bronze (Copper+Tin)
- Dark yellow colour
- More endurance than brass
- Very corrosion resistant
- Good sonority
- Very fluid when melting, good for molding.


Aluminum, Copper & Magnesium
- Lightness and more endurance than pure aluminum.


Magnesium & Aluminum
- This alloy is more resistant than each metal.


Titanium & Aluminum
- Cheaper than pure titanium parts.


Aluminium and aluminium alloys
Al and its alloys are characterized by :
- relatively low density (2.7 g/cm3 as compared to 7.9 g/cm3 for steel),
- high electrical and thermal conductivities,
- resistant to corrosion in some common environments, including the ambient atmosphere.
- Many of these alloys are easily formed due to high ductility;
- thin aluminum foil sheet (of relatively pure material) may be rolled.
- its ductility is retained even at very low temperatures.
- White shiny colour
- Light and good endurance
- No toxic Cheap Stainless
The chief limitation:
- low melting temperature [660°C],
- restricts the maximum temperature at which it can be used.
- The mechanical strength of aluminum may be enhanced by cold work and by alloying;
- However, both processes tend to diminish resistance to corrosion.
- Principal alloying elements include copper, magnesium, silicon, manganese, and zinc.


- Non heat-treatable alloys consist of a single phase,
- for which an increase in strength is achieved by solid-solution strengthening.
- Rendered heat treatable
- capable of being precipitation hardened as a result of alloying.
- In several of these alloys precipitation hardening is due to the precipitation of two elements other than aluminum, to form an intermetallic compound such as MgZn2.
Aluminium Composite Panel
Structural section of aluminum composite panel
- Protective film
- PVDF clear coating
- PVDF top coating
- PVDF primer
- Chromate treatment
- Aluminum skin
- Chromate treatment
- Bonding lamination layer
- Nontoxic PE core material
- Optional fire resistant and nontoxic PE core material
- Optional back coating
Characteristics of aluminium composite panel (ACP)
Economy – Due to our adoption of high quality coatings, our aluminum composite panels are dispensed with frequent maintenance, thus saving a lot on maintenance cost. Hence, our composite panels are good decorative building material for high-rise buildings.
Durability – Fluorocarbon coated aluminum composite panels are characterized by long service life, good weather resistance, high heat resistance and wear resistance, and good resistance to pollutions, and more.
Flatness – Our aluminum composite panels are manufactured with smooth and even surface, which can satisfy people’s high demand on the appearance of modern constructions.
Light weight – By adopting light-weight aluminium alloy and superior quality plastics as primary raw material, aluminum composite panel is lighter than other types of decorative construction material. Hence, it is easy to install and needs shorter installation period.
Easy installation – Our aluminium composite panels are adaptable to various decoration requirements, because they are easy to be cut, grooved and bended.
Fireproofing – The exterior aluminium sheet of aluminum composite panels can effectively protect interior PE core material, because it can prevent heat conduction at the initial stage of combustion. The flame resistance of our aluminum plastic composite panels has reached international standard.
Design performance – Aluminum composite panels can be made into different colors and styles, thus satisfying the diversified design requirements of architects.
Eco-friendliness – The surface coating is durable and has no changes, which produces no pollutants.
Application of Aluminium Composite Panel
- Wall and interior decoration of air ports, docks, stations, metros, hotels, restaurants, recreation places, top-grade residence, villas, office, and many other buildings.
- Big billboards, shop windows, roadside news kiosks, bookstalls, telephone booths, traffic sentry boxes and filling stations.
- Internal walls, ceilings, compartments, kitchens, toilets, etc.
- Decoration for shops, cabinets, pillars, furniture, trains, automobiles, ships and carriages, etc.
- Renovation and reconstruction of old buildings.
- Cleaning and dust control projects.
PVDF Aluminium Composite Panel
The PVDF aluminum composite panel consists of two sheets of corrosion resistant aluminum permanently bonded to polyethylene core material. The front aluminum coats PVDF (Fluor resin) paint. Because of its excellent weather proof and other features, this aluminium composite material becomes a very popular cladding material in the world.
Specifications
- Panel thickness 3mm,4mm.5mm,6mm
- Panel width 1000mm,1220mm,1250mm,1350mm,1500mm,1570mm
- Panel length 2440mm-5800mm,or per customer’s request
- Alu skin thickness 0.25mm,0.30mm,0.35mm,0.40mm,0.45mm,0.50mm
- Standard size 1220(W) ×2440(L)mm
- paint polyester coating
- core Normal PE core
Fluoropolymers have been used in a multitude of high performance coating applications for over thirty five years. Poly(vinylidene fluoride) (PVDF) has been used especially in architectural applications, where both excellent appearance and substrate protection must be maintained over a very long period of time.
PVDF coatings can be applied by conventional coil or spray coating techniques, and baked at temperatures of 230-250 ºC.
Fireproof Aluminium Composite Panel
Our fireproof aluminum composite panel is made of fireproof plastic core with two layers of aluminum sheets. This new decorative material comes with B1 fireproof grade according to GB8624 test. It is green material, as there is no harmful gas during burning.

Mirror Finished Aluminium Composite Panel
The front side of the mirror finished aluminum composite panel is treated like a mirror. The panel looks more beautiful and widely used in interior decoration, kitchen, especially in ceiling and household appliance and furniture etc..
Specification
- Panel thickness 3mm,4mm.5mm,6mm
- Panel width 1220mm
- Panel length 2440mm-5800mm,or per customer’s request
- Alu skin thickness 0.30mm,0.40mm
- Standard size 1220(W) ×2440(L)mm
- core Normal PE core
PE Aluminium Composite Panel
The PE aluminum composite panel consists of two sheets of corrosion resistant aluminum permanently bonded to polyethylene core material. The front aluminum coats PE (Polyester) paint. Due to its excellent flexibility and sound insulation performance, etc., this aluminum composite panel is widely used as interior decoration materials all around world.
Specification :
- Panel thickness :3mm,4mm.5mm,6mm
- Panel width: 1000mm,1220mm,1250mm,1350mm,1500mm,1570mm
- Panel length: 2440mm-5800mm,or per customer’s request
- Alu skin thickness 0.15mm,0.21mm,0.30mm,0.40mm, 0.50mm
- Standard size 1220(W) ×2440(L)mm
- Paint Polyester coating
- Core Normal PE core

Manufacturing of Aluminium
Five major steps in recovering alumina from bauxite.
STEP 1- Crushing and Grinding:
Alumina recovery begins by passing the bauxite through screens to sort it by size. It is then crushed to produce relatively uniformly sized material. The ore is then fed into large grinding mills and mixed with a caustic soda solution (sodium hydroxide) at high temperature and pressure.
The grinding mill rotates like a huge drum while steel rods – rolling around loose inside the mill – grind the ore to an even finer consistency. The process is a lot like a kitchen blender only much slower and much larger. The material finally discharged from the mill is called slurry. The resulting liquor contains a solution of sodium aluminate and undissolved bauxite residues containing iron, silicon, and titanium. These residues – commonly referred to as “red mud” – gradually sink to the bottom of the tank and are removed.
Four tons of bauxite are required to produce 2 tons of alumina.

STEP 2-Digesting:
The slurry is pumped to a digester where the chemical reaction to dissolve the alumina takes place. In the digester the slurry – under 50 pounds per square inch pressure – is heated to 300 °Fahrenheit (145 °Celsius). It remains in the digester under those conditions from 30 minutes to several hours.
More caustic soda is added to dissolve aluminum containing compounds in the slurry. Undesirable compounds either don’t dissolve in the caustic soda, or combine with other compounds to create a scale on equipment which must be periodically cleaned. The digestion process produces a sodium aluminate solution. Because all of this takes place in a pressure cooker, the slurry is pumped into a series of “flash tanks” to reduce the pressure and heat before it is transferred into “settling tanks.”
STEP 3-Settling:
Settling is achieved primarily by using gravity, although some chemicals are added to aid the process. Just as a glass of sugar water with fine sand suspended in it will separate out over time, the impurities in the slurry – things like sand and iron and other trace elements that do not dissolve – will eventually settle to the bottom.
The liquor at the top of the tank (which looks like coffee) is now directed through a series of filters. After washing to recover alumina and caustic soda, the remaining red mud is pumped into large storage ponds where it is dried by evaporation. The alumina in the still warm liquor consists of tiny, suspended crystals. However there are still some very fine, solid impurities that must be removed. Just as coffee filters keep the grounds out of your cup, the filters here work the same way. The giant-sized filters consist of a series of “leaves” – big cloth filters over steel frames – and remove much of the remaining solids in the liquor. The material caught by the filters is known as a “filter cake” and is washed to remove alumina and caustic soda. The filtered liquor – a sodium aluminate solution – is then cooled and pumped to the “precipitators.”
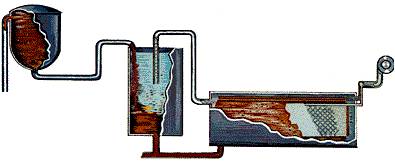
STEP 4-Precipitation:
Imagine a tank as tall as a six-story building. Now imagine row after row of those tanks called precipitators. The clear sodium aluminate from the settling and filtering operation is pumped into these precipitators. Fine particles of alumina – called “seed crystals” (alumina hydrate) – are added to start the precipitation of pure alumina particles as the liquor cools. Alumina crystals begin to grow around the seeds, then settle to the bottom of the tank where they are removed and transferred to “thickening tanks.” Finally, it is filtered again then transferred by conveyor to the “calcination kilns.”
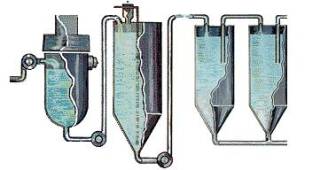
STEP 5-Calcination
Calcination is a heating process to remove the chemically combined water from the alumina hydrate. That’s why, once the hydrated alumina is calcined, it is referred to as anhydrous alumina. “Anhydrous” means “without water.”
From precipitation, the hydrate is filtered and washed to rinse away impurities and remove moisture. A continuous conveyor system delivers the hydrate into the calcining kiln. The calcining kiln is brick-lined inside and gas-fired to a temperature of 2,000 °F or 1,100 °C. It slowly rotates (to make sure the alumina dries evenly) and is mounted on a tilted foundation which allows the alumina to move through it to cooling eqipment. (Newer plants use a method called fluid bed calcining where alumina particles are suspended above a screen by hot air and calcined.)The result is a white powder like that shown below: pure alumina. The caustic soda is returned to the beginning of the process and used again.

At this point, the alumina is ready for conversion into aluminum at a smelter. Alumina is also used in making chemical and ceramics.

Converting Alumina to Aluminum
Smelting: In 1886, Charles Hall of the USA and Paul L.T. Heroult of France, made the same discovery – molten cryolite (a sodium aluminum fluoride mineral) could be used to dissolve alumina and the resulting chemical reaction would produce metallic aluminum. The Hall-Heroult process remains in use today.
Two tons of alumina are required to make one ton of aluminum.
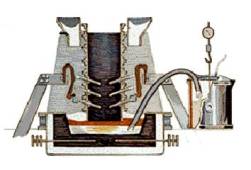
Converting Alumina to Aluminum : The Hall-Heroult process
The Hall-Heroult process takes place in a large carbon or graphite lined steel container called a ” reduction pot”. In most plants, the pots are lined up in long rows, called potlines.
The key to the chemical reaction necessary to convert the alumina to metallic aluminum is the running of an electrical current through the cryolite/alumina mixture. The process requires the use of direct current (DC) – not the alternating current (AC) used in homes. The immense amounts of power required to produce aluminum is the reason why aluminum plants are almost always located in areas where affordable electrical power is readily available. Some experts maintain that one percent of all the energy used in the United States is used in the making of aluminum.
The electrical voltage used in a typical reduction pot is only 5.25 volts, but the amperage is VERY high – generally in the range of 100,000 to 150,000 amperes or more. The current flows between a carbon anode (positively charged), made of petroleum coke and pitch, and a cathode (negatively charged), formed by the thick carbon or graphite lining of the pot.
When the electric current passes through the mixture, the carbon of the anode combines with the oxygen in the alumina. The chemical reaction produces metallic aluminum and carbon dioxide. The molten aluminum settles to the bottom of the pot where it is periodically syphoned off into crucibles while the carbon dioxide – a gas – escapes. Very little cryolite is lost in the process, and the alumina is constantly replenished from storage containers above the reduction pots.
The metal is now ready to be forged, turned into alloys, or extruded into the shapes and forms necessary to make appliances, electronics, automobiles, airplanes cans and hundreds of other familiar, useful items.
Aluminum is formed at about 900 °C, but once formed has a melting point of only 660 °C. In some smelters this spare heat is used to melt recycled metal, which is then blended with the new metal. Recycled metal requires only 5 per cent of the energy required to make new metal. Blending recycled metal with new metal allows considerable energy savings, as well as the efficient use of the extra heat available. When it comes to quality, there is no difference between primary metal and recycled metal.
The smelting process required to produce aluminum from the alumina is continuous the potline is usually kept in production 24 hours a day year-round. A smelter cannot easily be stopped and restarted. If production is interrupted by a power supply failure of more than four hours, the metal in the pots will solidify, often requiring an expensive rebuilding process. The cost of building a typical, modern smelter is about $1.6 billion.
Most smelters produce aluminum that is 99.7% pure – acceptable for most applications. However, super pure aluminum (99.99%) is required for some special applications, typically those where high ductility or conductivity is required. It should be noted that what may appear to be marginal differences in the purities of smelter grade aluminum and super purity aluminum can result in significant changes in the properties of the metal.
About 6.2 kWH (kilowatt hours) of electricity is required to produce one pound of aluminum from alumina.
Aluminium and aluminium alloys
Duralumin – 94% Al + 4% Cu+ 0.5% Mg + 0.5% Mn + 0.5% Si+ 0.5% Fe Used in aircraft and automobile
Aldural – It is duralumin with thin coating of pure aluminium Used in under water application.
Aluminum Bronze – 10 % to 22%Al + 90% to 78% Cu Used in die casting and pump rods.
Y-alloy – 92.5% Al + 4% Cu+ 1.5% Mg + 2% Ni Used for making pistons , cylinder heads and gear boxes.
Factors for selecting are:
- High strength to weight ratio
- Resistance to corrosion
- High thermal and electrical conductivity
- Ease of machinability
- Non-magnetic
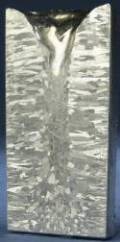
Cast Structure of Al Ingot
Copper and copper alloys
- Copper alloys have electrical and mechanical properties, corrosion resistance, thermal conductivity and wear resistance.
- Applications are electronic components, springs and heat exchangers.
- Brass is an alloy of copper and zinc.
- Bronze is an alloy of copper and tin.
- Have been utilized in quite a variety of applications since antiquity.
- Unalloyed copper is so soft and ductile that it is difficult to machine;
- it has an almost unlimited capacity to be cold worked.
- it is highly resistant to corrosion in diverse environments including the ambient atmosphere, seawater, and some industrial chemicals.
- The mechanical and corrosion-resistance properties of copper may be improved by alloying.
- Most copper alloys cannot be hardened or strengthened by heat-treating procedures;
- Only cold working and/or solid-solution alloying can be utilized to improve these mechanical properties.


Copper Properties
- Red colour
- Excellent thermal and electric conductor
- Corrosion resistant
- Good welding
- Very ductile and malleable
Copper Alloys: brasses
Cartridge Brass :
70% Cu+ 30% Zn Used in Cartridges , tubes , springs etc.
Muntz Metal
60% Cu+ 40% Zn, Used in Casting , Condenser Tubes etc.
Naval Brass
60% Cu+ 39% Zn+ 1% Sn Used in Marine and engineering casting like condeser tubes , pump parts , motor boat shafting.
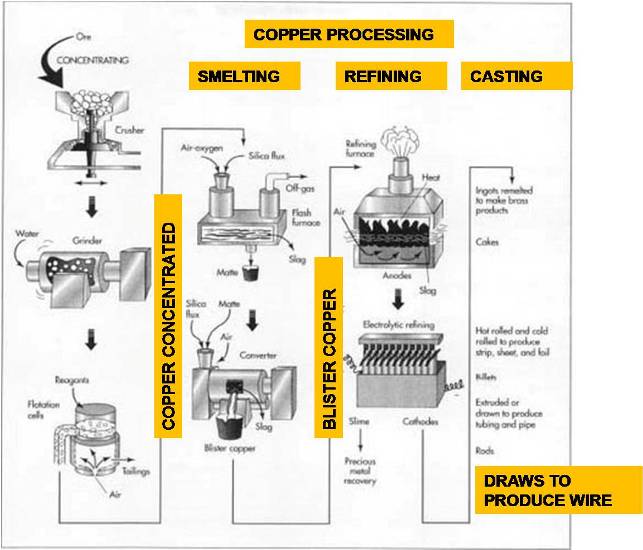
The Manufacturing Process : Copper
Pure copper is rarely found in nature, but is usually combined with other chemicals in the form of copper ores. The most common are known as sulfide ores in which the copper is chemically bonded with sulfur. Others are known as oxide ores, carbonate ores, or mixed ores depending on the chemicals present. Many copper ores also contain significant quantities of gold, silver, nickel, and other valuable metals, as well as large quantities of commercially useless material.
The most common sulfide ore is Chalcopyrite, CuFeS 2 , also known as copper pyrite or yellow copper ore.
Chalcocite, Cu 2 S, is another sulfide ore.
Cuprite, or red copper ore, Cu 2 O, is an oxide ore.
Malachite, or green copper ore, Cu(OH) 2 •CuCO 3 , is an important carbonate ore, as is Azurite, or blue copper carbonate, Cu(OH) 2 •2CuCO 3 .
Other ores include tennantite, boronite, chrysocolla, and atacamite.
Mining
Most sulfide ores are taken from huge open-pit mines by drilling and blasting with explosives. In this type of mining, the material located above the ore, called the overburden, is first removed to expose the buried ore deposit. This produces an open pit that may grow to be a mile or more across. A road to allow access for equipment spirals down the interior slopes of the pit.
The exposed ore is scooped up by large power shovels capable of loading 500-900 cubic feet (15-25 cubic meters) in a single bite. The ore is loaded into giant dump trucks, called haul trucks, and is transported up and out of the pit.
Concentrating
The copper ore usually contains a large amount of dirt, clay, and a variety of non-copper bearing minerals. The first step is to remove some of this waste material. This process is called concentrating and is usually done by the flotation method.
The ore is crushed in a series of cone crushers. A cone crusher consists of an interior grinding cone that rotates on an eccentric vertical axis inside a fixed outer cone. As the ore is fed into the top of the crusher, it is squeezed between the two cones and broken into smaller pieces.
The crushed ore is then ground even smaller by a series of mills. First, it is mixed with water and placed in a rod mill, which consists of a large cylindrical container filled with numerous short lengths of steel rod. As the cylinder rotates on its horizontal axis, the steel rods tumble and break up the ore into pieces about 0.13 in (3 mm) in diameter. The mixture of ore and water is further broken up in two ball mills, which are like a rod mill except steel balls are used instead of rods. The slurry of finely ground ore that emerges from the final ball mill contains particles about 0.01 in (0.25 mm) in diameter.
The slurry is mixed with various chemical reagents, which coat the copper particles. A liquid, called a frother, is also added. Pine oil or long-chain alcohol are often used as frothers. This mixture is pumped into rectangular tanks, called flotation cells, where air is injected into the slurry through the bottom of the tanks. The chemical reagents make the copper particles cling to the bubbles as they rise to the surface. The frother forms a thick layer of bubbles, which overflows the tanks and is collected in troughs. The bubbles are allowed to condense and the water is drained off. The resulting mixture, called a copper concentrate, contains about 25-35% copper along with various sulfides of copper and iron, plus smaller concentrations of gold, silver, and other materials. The remaining materials in the tank are called the gangue or tailings. They are pumped into settling ponds and allowed to dry. The process of extracting copper from copper ore varies according to the type of ore and the desired purity of the final product. Each process consists of several steps in which unwanted materials are physically or chemically removed, and the concentration of copper is progressively increased.
Smelting
Once the waste materials have been physically removed from the ore, the remaining copper concentrate must undergo several chemical reactions to remove the iron and sulfur. This process is called smelting and traditionally involves two furnaces as described below. Some modern plants utilize a single furnace, which combines both operations.
The copper concentrate is fed into a furnace along with a silica material, called a flux. Most copper smelters utilize oxygen-enriched flash furnaces in which preheated, oxygen-enriched air is forced into the furnace to combust with fuel oil. The copper concentrate and flux melt, and collect in the bottom of the furnace. Much of the iron in the concentrate chemically combines with the flux to form a slag, which is skimmed off the surface of the molten material. Much of the sulfur in the concentrate combines with the oxygen to form sulfur dioxide, which is exhausted from the furnace as a gas and is further treated in an acid plant to produce sulfuric acid. The remaining molten material in the bottom of the furnace is called the matte. It is a mixture of copper sulfides and iron sulfides and contains about 60% copper by weight.
Types Of Copper Tube .
There are six standard types of copper tube:
- Type K (green), is the heaviest wall and is used in higher pressure applications;
- Type L (blue), has a medium wall and is used in mid-pressure applications;
- Type M (red), has a lighter wall that Type L and is used in low-pressure applications;
- DWV (for Drain Waste Vent, yellow), has the thinnest walled and used in drain, waste, vent applications with little to no pressure involved;
- ACR (for Air Conditioning/Refrigeration, blue) used primarily in HVAC/R applications;
- OXY/MED (blue, green) used for medical gas applications.

Magnesium and magnesium alloys
- Magnesium (Mg) is the lightest metal.
- Alloys are used in structural and non-structural applications.
- Typical uses of magnesium alloys are aircraft and missile components.
- Also has good vibration-damping characteristics.
- Compared with aluminum, magnesium is approximately two-thirds of its weight.
PROPERTIES OF MAGNESIUM :
- Specific gravity 1.74
- Melts at 651°C
- Boiling Point is 1110°C
- It is silver white processing high lustre.
Magnesium Wrought alloys
Magnesium alloys for wrought products are classified as non-heat treatable and heat treatable. AZ80A and ZK60A are heat treated by aging the material as fabricated. The strengths are increased substantially but with loss of elongation on aging. M1A and AZ31B sheet are supplied as hot rolled, as cold rolled, and annealed.
The principal wrought manufactures which are commercially available in magnesium alloys include sheet and other flat rolled products, extrusions (bars, rods, solid shapes, hollow shapes, and tubing), forgings, screw-machine stock, and impact extrusions.



Burns easily when being machined
Magnesium Wheel
Used with Aluminium to make lightweight alloys. Place pieces of magnesium adjacent to buried steel pipelines and water tanks. The magnesium reacts chemically to the elements found in the earth and prevents corrosion of the steel.

Nickel and nickel alloys
Nickel has been used in alloys that date back to the dawn of civilization. Chemical analysis of artifacts has shown that weapons, tools, and coins contain nickel in varying amounts.
Nickel in elemental form or alloyed with other metals and materials has made significant contributions to our present-day society and promises to continue to supply materials for an even more demanding future.
Nickel is a versatile element and will alloy with most metals. Complete solid solubility exists between nickel and copper. Wide solubility ranges between iron, chromium, and nickel make possible many alloy combinations.
- Nickel (Ni) has strength, toughness, and corrosion resistance to metals.
- Used in stainless steels and nickel-base alloys.
- Alloys are used for high temperature applications, such as jet-engine components and rockets.
- PROPERTIES OF NICKEL :
- Specific gravity 8.90
- Melts at 1452°C
- Boiling Point is 2900°C
- It is grayish white lustrous metal
Manganese & alloys
- Manganese is primarily produced from the mineral pyrolusite (MnO2), which, on average, contains more than 50% manganese.
- Manganese is an extremely brittle and hard, silvery-grey metal. The twelfth most abundant element in the earth’s crust, manganese increases strength, hardness and wear resistance when alloyed in steel.
- Excellent formability , welding and corrosion resistance.
- PROPERTIES :
- Density: 7.21 g/cm³
- Melting Point: 1246°C
- Boiling Point: 2061 °C
- Mohs Hardness: 6
Tin & alloys
Tin was one of the first metals known to man. Throughout ancient history, various cultures recognized the virtues of tin in coatings, alloys and compounds, and use of the metal increased with advancing technology. Today, tin is an important metal in industry even though the annual tonnage used is much smaller than those of many other metals. One reason for the small tonnage is that, in most applications, only very small amounts of tin are used at a time.
- It is very costly metal and does not occur abundantly in nature
- The most important ore is tinstone or cassiterite ( SnO3 )
- Used for preparing solder material , because it melts easily and does not corrode
- It is used for coating iron and steel sheets
- Used in Bronze
- Bluish white shiny colour
- Soft, ductile and malleable
- Corrosion resistant, rustfree
- Low melting point. Melts at 232°C


Pewter – Pewter is a tin-base white metal containing antimony and copper. Originally, pewter was defined as an alloy of tin and lead, but to avoid toxicity and dullness of finish, lead is excluded from modern pewter. These modem compositions contain 1 to 8% antimony and 0.25 to 3.0% copper.
Dental alloys – Dental alloys for making amalgams contain silver, tin, mercury, and some copper and zinc. The copper increases hardness and strength and the zinc acts as a scavenger during alloy manufacture, protecting major constituents from oxidation. Most dental alloys presently available contain 25 to 27% tin and consist mainly of the inter-metallic compound Ag and Sn. When porcelain veneers are added to gold alloys for high-grade dental restoration, 1% tin is added to the gold alloy to ensure bonding with the porcelain.
Zinc & alloys
- The most important ore is zinc oxide ( ZnO), zinc carbonate ( ZnCO3 )
- Used in electric bells and for preparing printing block
- Used for making brass
- The mechanical properties of these zinc alloys make them attractive substitutes for cast iron and copper alloys in many structural and pressure-tight applications. Because zinc is less costly than copper, these zinc alloys have a distinct cost advantage over copper-base alloys. The ease of machining of zinc and its inherent corrosion resistance give it advantages over cast iron.
- Melts at 419°C
- It is brittle at ordinary temp.
- Crystalline blue white metal
- Resist corrosion


Wrought Zinc & alloys
Wrought zinc and zinc alloys may be obtained as rolled strip, sheet and foil; extruded rod and shapes; and drawn rod and wire. These metals exhibit good resistance to corrosion in many types of service, and because the corrosion products that may form on them are white, other materials are not stained by them.
Wrought zinc has chemical characteristics particularly adapted to certain uses, such as dry batteries and photoengraver`s plate, and offers combinations of desirable physical and mechanical properties at relatively low cost. In common with many other metals and alloys, wrought zinc creeps under constant loads that are substantially less than its ultimate strength; that is, wrought zinc does not have clearly defined elastic module, and hence creep data from service tests must be used in designing for strength and rigidity under conditions of continuos stress.
Titanium and Titanium alloys
- Since the introduction of titanium and titanium alloys in the early 1950s, these materials have in a relatively short time become backbone materials for the aerospace, energy, and chemical industries.
- The combination of high strength-to-weight ratio, excellent mechanical properties, and corrosion resistance makes titanium the best material choice for many critical applications.
- Today, titanium alloys are used for demanding applications such as static and rotating gas turbine engine components.
- Some of the most critical and highly-stressed civilian and military airframe parts are made of these alloys.
- Very expensive
- Endurance like steel but quite lighter.
- Biocompatible

Lead and Lead alloys
- Lead was one of the first metals known to man. Probably the oldest lead artifact is a figure made about 3000 BC. All civilizations, beginning with the ancient Egyptians, Assyrians, and Babylonians, have used lead for many ornamental and structural purposes.
- Lead’s chemical symbol comes from the Latin word for waterworks, plumbum.
- Many magnificent buildings erected in the 15th and 16th centuries still stand under their original lead roofs.
- Grey colour
- Very soft
- Low melting point
- Ductile and malleable
- Very toxic


The properties of lead that make it useful in a wide variety of applications are density, malleability, lubricity, flexibility, electrical conductivity, and coefficient of thermal expansion, all of which are quite high; and elastic modulus, elastic limit, strength, hardness, and melting point, all of which are quite low. Lead also has good resistance to corrosion under a wide variety of conditions. Lead is easily alloyed with many other metals and casts with little difficulty.
The high density of lead (11.35 g/cm3, at room temperature) makes it very effective in shielding against x-rays and gamma radiation. The combination of high density, high limpness (low stiffness), and high damping capacity makes lead an excellent material for deadening sound and for isolating equipment and structures from mechanical vibrations.
Refractory metals
- Refractory metals have a high melting point and retain their strength at elevated temperatures.
- Applications are electronics, nuclear power and chemical industries.
- Molybdenum, columbium, tungsten, and tantalum are referred to as refractory metal.
Surface Hardening Methods
- Flame hardening
- Induction heating
- High-frequency resistance heating
- Electron beam heating
- Laser beam heating
Forum Threads
Study Notes PDF
Study notes of NONFERROUS METAL & ALLOY
construction material NON ferrous metal.pdf
Register in Front Desk Architects & Planners Forum to download above PDF
Disclaimer
Information on this site is purely for education purpose. The materials used and displayed on the Sites, including text, photographs, graphics, illustrations and artwork, video, music and sound, and names, logos, IS Codes, are copyrighted items of respective owners. Front Desk is not responsible and liable for information shared above.
References
Books
Brady, George S., Henry R. Clauser, and John A. Vaccari. Materials Handbook. McGraw-Hill, 1997. Heiserman, David L. Exploring Chemical Elements and Their Compounds. TAB Books, 1992. Hombostel, Caleb. Construction Materials. John Wiley and Sons, Inc., 1991. Kroschwitz, Jacqueline I. and Mary Howe-Grant, ed. Encyclopedia of Chemical Technology. John Wiley and Sons, Inc., 1993. Stwertka, Albert. A Guide to the Elements. Oxford University Press, 1996.
Periodicals
Baum, Dan and Margaret L. Knox. “We want people who have a problem with mine wastes to think of Butte.” Smithsonian (November 1992): 46-52, 54-57. Shimada, Izumi and John F. Merkel. “Copper-Alloy Metallurgy in Ancient Peru.” Scientific American (July 1991): 80-86.
External Links
http://www.copper.org .
http://www.intercorr.com/periodic/29.htm .
http://innovations.copper.org/innovations.html .
http://www.rocksandminerals.com/aluminum/process.htm