- FORUM
- PROJECTS
- ABOUT US
- RESOURCES
- CONTACT US
- FORUM
- PROJECTS
- ABOUT US
- RESOURCES
- CONTACT US
Lime as Construction Material
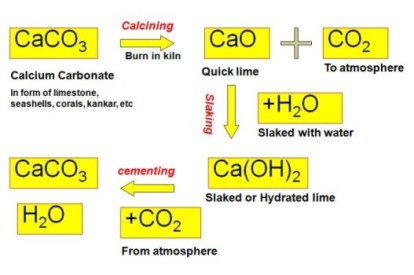
Lime is one of man’s oldest and most vital chemicals. The ancient Romans used lime in building and road construction, uses which continue to the present day. From earliest times, lime has been made by heating limestone (calcium carbonate) to high temperatures. This process, known as calcining, results in quicklime, or calcium oxide. Hydrated lime (calcium hydroxide) is produced by reacting quicklime with sufficient water to form a dry, white powder.
The cycle
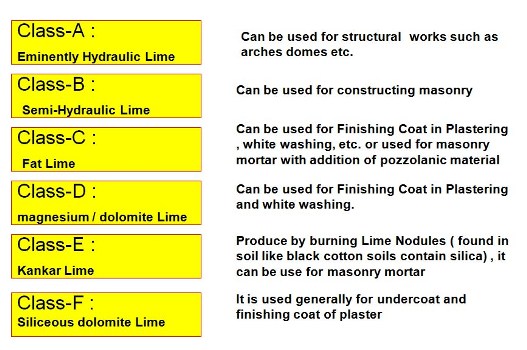
Classification of Lime IS 712-1973
Revival of lime
During the last 25 years, Lime has enjoyed a steady revival for repairs to historic buildings. The soft, porous and flexible nature of lime mortars and plasters is now universally recognized as being vital to maintain the traditional breathing performance of old buildings. In addition, an increasing number of architects, engineers, surveyors and builders are now beginning to realise that lime has many benefits to offer in new construction.
Lime KILN
A lime kiln is a kiln used to produce quicklime by the calcination of limestone (calcium carbonate). The chemical equation for this reaction is:
CaCO3 + heat = CaO + CO2
This reaction takes place at 900°C (at which temperature the partial pressure of CO2 is 1 atmosphere), but a temperature around 1000°C (at which temperature the partial pressure of CO2 is 3.8 atmospheres) is usually used to make the reaction proceed quickly. Excessive temperature is avoided because it produces unreactive, “dead-burned” lime.
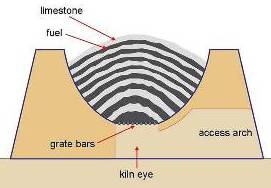
Old lime kiln
The common feature of early kilns was an egg-cup shaped burning chamber, with an air inlet at the base (the “eye”), constructed of brick. Limestone was crushed (often by hand) to fairly uniform 20-60 mm (1 to 2.5 inch) lumps – fine stone was rejected. Successive dome-shaped layers of coal and limestone were built up in the kiln on grate bars across the eye. When loading was complete, the kiln was kindled at the bottom, and the fire gradually spread upwards through the charge. When burnt through, the lime was cooled and raked out through the base. Fine coal ash dropped out and was rejected with the “riddlings”.

Only lump stone could be used, because the charge needed to “breathe” during firing. This also limited the size of kilns and explains why kilns were all much the same size. Above a certain diameter, the half-burned charge would be likely to collapse under its own weight, extinguishing the fire. So kilns always made 25-30 tonnes of lime in a batch.
19th century limekilns at Froghall
Typically the kiln took a day to load, three days to fire, two days to cool and a day to unload, so a one-week turnaround was normal. The degree of burning was controlled by trial and error from batch to batch by varying the amount of fuel used. Because there were large temperature differences between the center of the charge and the material close to the wall, a mixture of under-burned (i.e. high loss on ignition), well-burned and dead-burned lime was normally produced. Typical fuel efficiency was low, with 0.5 tonnes or more of coal being used per tonne of finished lime (15 MJ/kg).

A preserved lime kiln in Burgess Park, London

Modern lime kiln-Shaft kilns
Shaft kilns
The theoretical heat (the standard enthalpy) of reaction required to make high-calcium lime is around 3.15 MJ per kg of lime, so the batch kilns were only around 20% efficient. The key to development in efficiency was the invention of continuous kilns, avoiding the wasteful heat-up and cool-down cycles of the batch kilns. The first were simple shaft kilns, similar in construction to blast furnaces. These are counter-current shaft kilns. Modern variants include regenerative and annular kilns. Output is usually in the range 100-500 tonnes per day.
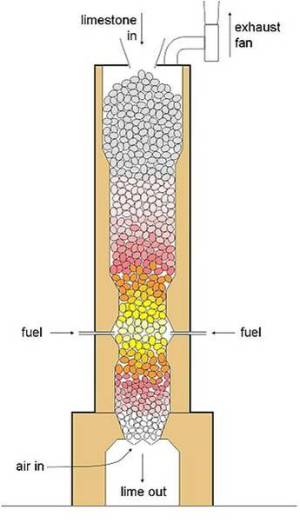
Modern lime kiln- Rotary kilns
Rotary kilns started to be used for lime manufacture at the start of the 20th century and now account for a large proportion of new installations. The early use of simple rotary kilns had the advantages that a much wider range of limestone size could be used, from fines upwards, and undesirable elements such as sulfur can be removed. On the other hand, fuel consumption was relatively high because of poor heat exchange compared with shaft kilns, leading to excessive heat loss in exhaust gases. Modern installations partially overcome this disadvantage by adding a preheater, which has the same good solids/gas contact as a shaft kiln, but fuel consumption is still somewhat higher.
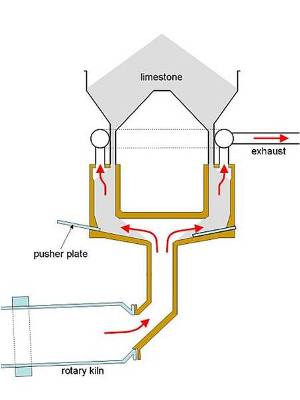
In the design shown, a circle of shafts (typically 8-15) is arranged around the kiln riser duct. Hot limestone is discharged from the shafts in sequence, by the action of a hydraulic “pusher plate”. Kilns of 1000 tonnes per day output are typical.
Lime Mortar
Lime mortar is a type of mortar composed of lime, an aggregate such as sand, and water. It is one of the oldest known types of mortar, dating back to the 4th century BCE and widely used in Ancient Rome and Greece, when it largely replaced the clay and gypsum mortars common to Ancient Egyptian construction.
Lime mortar is used as an alternative to ordinary portland cement. It is made principally of lime (hydraulic, or non hydraulic), water and an aggregate such as sand.
Hydraulic limes
Hydraulic limes set under water and non-hydraulic limes need air to carbonate and therefore set. Modern non hydraulic lime mortars are produced from lime derived from high calcium lime stones. These lime stones are burnt in kilns producing quick lime for other industrial uses other than building.
In the past, countless kilns all over the country burnt lime stones of varying qualities – many of these lime stones containing impurities making them unsuitable for today’s industrial processes but eminently suitable for building due to their varying degrees of hydraulicity.
All but the kilns burning pure lime stones ceased production as ordinary portland cement gained widespread use. However a very small number of kilns are producing hydraulic lime for the building industry to standards which are now expected of any building material.
Non-hydraulic limes
Non-hydraulic lime is primarily composed of calcium hydroxide (generally greater than 95%). Non-hydraulic lime is produced by the heating of sufficiently pure limestone (calcium carbonate) to between 954° and 1066°C, driving off carbon dioxide, to produce quicklime (calcium oxide). As well as calcium based limestone, dolomitic limes can be produced which are based on calcium magnesium carbonate. This is done in a lime kiln. The quicklime is then – thoroughly mixed with water to produce lime putty (calcium hydroxide), or with less water to produce dry hydrated lime.
The slaking process involved in creating a lime putty is an exothermic vigorous reaction which initially creates a liquid of a cream consistency. This then has to be matured for between 2 to 3 months – depending upon environmental conditions – to allow time for it to condense and mature into a lime putty. A matured lime putty displays a physical property known as “thixotropic” which means that when a lime putty is physically agitated it changes from a putty into a more liquid state. This aids its use for mortars as it makes a mortar easier to work with and apply. If left to stand following agitation a lime putty will slowly revert from a thick liquid back to a putty state. It is always advised that a lime mortar should be “knocked up” prior to its use.
Hydrated lime and lime putty
Non-hydraulic lime is produced in two forms:
- hydrated lime
A frequent source of confusion regarding lime mortar stems from the similarity of the terms hydraulic and hydrated, however the two terms, in this context, have different meanings. Hydrated lime is any lime other than quicklime, so can refer to either hydraulic (hardens underwater) or non-hydraulic (doesn’t harden underwater) lime. Stored lime putty is always non-hydraulic (since hydraulic putty sets quickly after mixing) and, as the name suggests, lime putty is in the form of a putty made from just lime and water.
If the quicklime is slaked with an excess of water then putty or slurry is produced. If less water is used, then the result is a dry material (any excess water escaping as steam during heating). This is ground to make hydrated lime.
- lime putty.
Hydrated non-hydraulic lime can be mixed with water to form lime putty. Before use it is usually left in the absence of carbon dioxide (usually under water) to mature.
Putty can be matured for anything from 24 hours to many years, an increased maturation time improving the quality of the putty. There is however an argument that a lime putty which has been matured for an extended period eg over 12 months, becomes so stiff that it is less workable.
There is some dispute as to the comparative quality of putty formed from hydrated lime compared to that produced as putty at the time of slaking. It is generally agreed that the latter is preferable.
A hydrated lime will produce a material which is not as “fatty” and often due to lengthy and poor storage, the resulting lime produced by hydrated lime will exhibit longer carbonation periods as well as lower compressive strengths.
Hydraulic Lime
- In the context of lime or cement, the term ‘hydraulic’ means to ‘harden under water’.
- Hydraulic lime can be considered, in terms both of properties and manufacture, as part-way between non-hydraulic lime and OPC. The limestone used contains sufficient quantities of clay and/or silica. The resultant product will contain dicalcium silicate but unlike OPC not tricalcium silicate.
- It is slaked enough to convert the calcium oxide to calcium hydroxide but not with sufficient water to react with the dicalcium silicate. It is this dicalcium silicate which in combination with water provides the setting properties of hydraulic lime.
- Aluminium and magnesium also produce a hydraulic set, and some pozzolans contain these elements.
Advantages of Lime
1. Lime Allows Buildings To Breathe
In the search by architects and conservators for building materials sympathetic to traditional construction, lime was found to be one of the most important. One of the reasons lime binders are promoted by the Society for the Protection of Ancient Buildings for repairs is because they are vapour permeable and allow buildings to breathe. This reduces the risk of trapped moisture and consequent damage to the building fabric
2. Lime Provides A Comfortable Environment
Porous and open textured materials such as lime plasters, help to stabilize the internal humidity of a building by absorbing and releasing moisture. This makes for a more comfortable environment and reduces surface condensation and mould growth.
3. The Use Of Lime Has Ecological Benefits
- Lime has less embodied energy than cement.
- Free lime absorbs carbon dioxide in the setting process of carbonation.
- It is possible to produce lime on a small scale.
- The gentle binding properties of lime enable full re-use of other materials.
- A very low proportion of quicklime will stabilize clay soils.
- Small quantities of lime can protect otherwise vulnerable, very low energy materials such as earth construction and straw bales.
4. Lime Binds Gently With Early Adhesion
The fine particle size of lime, far smaller than cement, is linked to the root meaning of the word lime, which is ‘sticky material’. Due to the fine particle size, lime mixes penetrate minute voids in the background more deeply than other materials. They bind gently and the stickiness gives good adhesion to other surfaces.
5. Lime Mortar Can Protect Adjacent Materials
Lime mortars with a high free lime content are porous and permeable. These characteristics allow lime mortars to protect adjacent materials by handling moisture movements through the building fabric and protecting them from harmful salts. Adjacent materials frequently affected this way include timber and iron as well as stone and brick masonry.
6. Lime Renders Can Assist Drying Out By Evaporation
Dense and impermeable renders can trap moisture within the building fabric. Trapped moisture is often the agent for various decay mechanisms. Dense renders used in conjunction with softer materials or on weaker backgrounds can cause serious problems by creating local stresses. High calcium lime renders allow evaporation and reduce the risk of trapped moisture and decay. In simple terms, the greater the extent of pure lime and permeability the better this is for the building. This needs to be balanced with durability, however, and some reduction in permeability may be necessary to obtain adequate weathering qualities, hence the advantage of feebly hydraulic limes for external use.
7. Lime Mixes Have Good Workability
The ability of a mortar or plaster to remain smooth and mouldable, even against the suction it may experience from porous building materials, is termed workability. Good workability greatly assists good workmanship, helping to achieve full joints with good bonding to the other materials. This is what makes lime based mixes such a pleasure to use. The workability provided by the lime allows the inclusion of widely graded and sharp aggregates in the mix. These enhance both the performance and the aesthetic of the finished work.
8. Lime Binders Can Be Durable And Have Stood The Test Of Time
When used carefully, lime is exceptionally durable. Caesar’s Tower at Warwick Castle has stood the test of time for over 600 years, and many cathedrals have stood longer. An outstanding example is the Pantheon Temple in Rome which has a lime concrete dome spanning over 43 metres (142 feet). This has survived for nearly 2000 years.
9. Lime Finishes Are Beautiful
The double refraction of light through calcite crystals give a unique aesthetic combining a soft texture with a lustre that has a liveliness and delight of its own. The graceful softness apparent in lime based materials is a visual indication of their intrinsic permeability, workability and soft binding properties. They can rapidly develop a rich patina which has a glowing translucent quality.

10. Lime Contributes To A Healthy Environment
Lime is caustic and has been extensively used, often in the form of limewash, for its disinfectant qualities. Lime is also used for water purification. Lime mortars, plasters, renders and limewash have been used to create hygienic surfaces and improve comfort conditions within buildings for thousands of years.
11. Self Healing
The nature of ground conditions and the elements are such that all buildings are subject to varying degrees of movement over time. When buildings made with lime are subject to small movements they are more likely to develop many fine cracks than the individual large cracks which occur in stiffer cement-bound buildings. Water penetration can dissolve the ‘free’ lime and transport it. As the water evaporates this lime is deposited and begins to heal the cracks. This process is called autogenous, or self healing.
12. Free Lime Encourages the Growth of Calcite Crystals
Calcite crystals are a different shape to those formed by the more complex compounds in hydraulic limes and cements. The crystals form in voids in lime rich environments. The growth of calcite crystals adds strength over time and generally provides a more open and permeable material than the denser eminently hydraulic and OPC mixes with little or no free lime.
13. Local Limes Enhance Regional Identity And Diversity
The diversity of limestone types provides variety and local distinctiveness. Different limes will vary in colour, texture and setting properties. Local limes have a regional identity, they give a sense of place and provide a continuous link with the local aesthetic. Local colour is the obvious example in respect of limewashes.
14. Disfiguring By Cement Can Be Avoided By The Use Of Lime
On site the temptation to use quick and easy solutions for short term gain can lead to long term problems. The attraction of using excess cement to be ‘safe’ is understandable if not desirable. The fact that it is plentiful, inexpensive and readily available adds to the problem. There is a high probability that over-strong and dense mixes that are not fit for purpose will be used in excess. The physical damage and unsightly aesthetic that results from this can be avoided by the use of lime.
15. Indefinite Shelf Life
Non-hydraulic limes have an indefinite shelf life when stored without access to air, usually as a putty under water or in sealed containers. In fact the quality of the putty improves the longer it is stored.
Field Test : Heat Of Hydration Of Quick Lime
This simple test can also be performed ‘in the field’ with easily portable items. Though it is primarily for comparing the reactivity of quicklimes, especially for monitoring the burning conditions in a small lime kiln, the maximum temperature reached through the exothermic (heat producing) reaction of quicklime with water is a good indicator of the quality of the lime, at least in terms of the available CaO. The rate at which the temperature rises is an indicator of how reactive it is.
Apparatus
- No. 7 mesh sieve (2.83 mm).
- Thermos flask.
- Thermometer reading to at least 100°C.
- Clock or watch with seconds hand.
- Scale to weigh 50 g to ± 0.5 g
- A pestle and mortar or other means of crushing the quicklime to pass the No.7 mesh.
Method
Take several lumps of fresh quicklime, break them with a hammer on a clean surface, cone and quarter to get a representative sample of small fragments. Grind 100–200 g of this with a pestle and mortar, so that it just passes through a No.7 mesh sieve. Into a thermos flask put 170 ml of water at the normal prevailing water temperature, which in tropical countries may be 23°C. Carefully weigh out 50.0 g of the No.7 mesh quicklime, put it into the thermos flask, start the stop watch and begin gently stirring the mixture. At one-minute intervals, record the temperature of the water and continue doing so for 24 minutes. Note the maximum temperature (and the time it was reached). By comparing the maximum temperature, and the hydration curve of temperature against time, with those obtained with samples of quicklime of known available lime content, the quality of the sample can be compared and an estimate made of its available CaO content, as well as its degree of reactivity. .
Testing Of Lime
Determination of available lime by the rapid sugar test (using hydrochloric acid)
Apparatus
• 300 ml flask •100 ml burette, with stand •Balance capable •No.100 mesh sieve. (0.15 mm).
Materials
•CO2 free distilled water, if available. • Hydrochloric acid • Methyl orange indicator. • Phenolphthalein indicator. • Sucrose – granulated sugar is satisfactory – 15 g.
Method
Take 0.5 g of 100 mesh lime and brush it into a 300 ml flask containing 20 ml of CO2 free distilled water and stopper the flask. Swirl and heat to boiling for 2 minutes. Add 150 ml of water and at least 15 g of sucrose. Stopper the flask, shake at intervals for 5 minutes and allow to stand for 30 minutes to 1 hour. Add 2 drops phenolphthalein, wash down stopper and sides of flask with distilled water, then titrate in the original flask with the standard HCl solution. Add about 90% of the estimated amount of acid before shaking the flask and then complete titration, with the final acid being fed slowly until the pink colour disappears.
Note the reading
1 ml of the acid solution is equivalent to 1% available lime expressed as CaO.
Loss on ignition test (LOI)
The LOI test. can be conducted at regular intervals during production to monitor the relative degree of calcination. It should be accompanied by a thorough visual inspection. It is also used in the testing stage to compare LOI of limestone from different deposits.
Apparatus:
- Container of fixed volume (20 or 50 litres), such as a bucket.
- Scale of sufficient size to weigh the above volume.
Method
- 1. Weigh container (Wb)
- 2. Weigh the container filled with a representative sample of limestone feed (Wf).
- 3. Weigh the container filled with a representative sample of quicklime lumps (Wa), Or Conduct the above weighing exercise several times (5 will suffice) with different batches of limestone feed and quicklime lumps to determine average figures for (Wf) and (Wa).
- 4. Calculate the % weight lost on ignition using the following formula:
(Wf -Wa)/(Wf-Wb) X 100 % weight lost in ignition (% LOI)
The %LOI can be compared with a standard LOI figure calculated under precise laboratory conditions to establish the relative degree of burning, or if this is not available the theoretical value can be used. The volume of quicklime which has been used in the weighing exercise must be inspected to determine to what degree the limestone is overburnt.
If firing is conducted correctly there should be no, or a very little, underburnt stone, but this should also be checked for.
Overburnt quicklime lumps can be distinguished by:
- a) A difference in colour compared to lightly burnt lumps.
- b) A relative difference in weight between lumps of approximately the same size. Overburnt material will be heavier than lightly burnt material.
- c) Shrinkage due to overburning may cause cracks to appear.
- d) When tapped lightly with a hammer overburnt lumps will produce a sharp ringing tone compared to the tone produced by a lightly burnt stone
Forum Threads
| IS: 712 -1984 – SPECIFICATION FOR BUILDING LIMES |
| What is limestone |
| Uses of limestone |
| The chemistry of limestone |
| Types of Limestone |
| Revival of lime |
| Lime |
Study Notes PDF
Study notes of Construction Material LIME useful for students of architecture
construction material-LIME.pdf
Register in Front Desk Architects & Planners Forum to download above PDF
Disclaimer
Information on this site is purely for education purpose. The materials used and displayed on the Sites, including text, photographs, graphics, illustrations and artwork, video, music and sound, and names, logos, IS Codes, are copyrighted items of respective owners. Front Desk is not responsible and liable for information shared above.
1 Comment
Hello (Front Desk Owner),
I was doing some research on (What Is Lime) and found the post (What Is Lime) on your blog.
In the article, I see that you linked to (other person’s resource). I like a lot of the methods that he mentioned and I’ve tried a few myself. Personally, I’ve found that this can work even better if you (link our article which is on the same topic).
I actually wrote a guide on this called (What Is Lime). It might be worth a mention on your blog. Here’s the link, in case you’re interested: (https://www.civilquery.com/what-is-lime/)
Either way, keep up the great work!